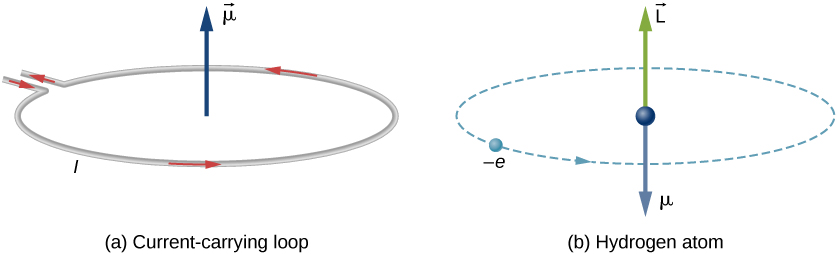
Magnetic Moment Physics Of Magnetic Resonance Imaging Magnetic Field Hydrogen Atom, PNG, 1233x874px, Magnetic Moment, Area,
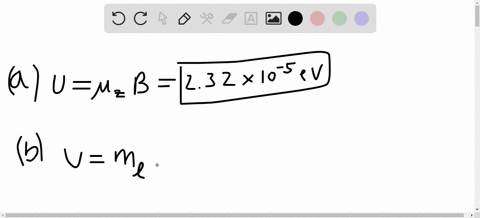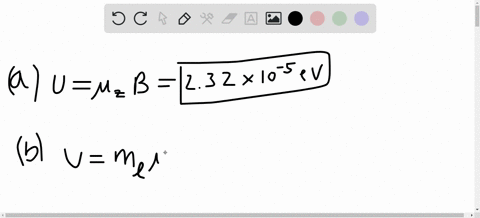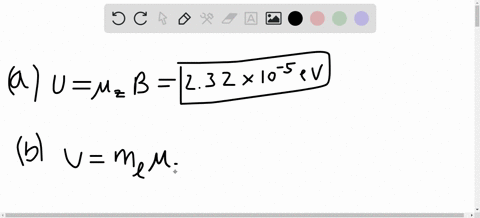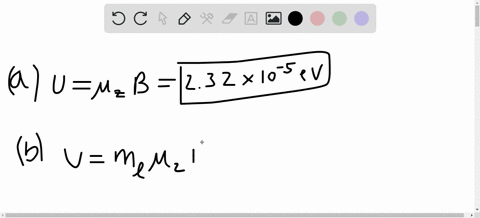
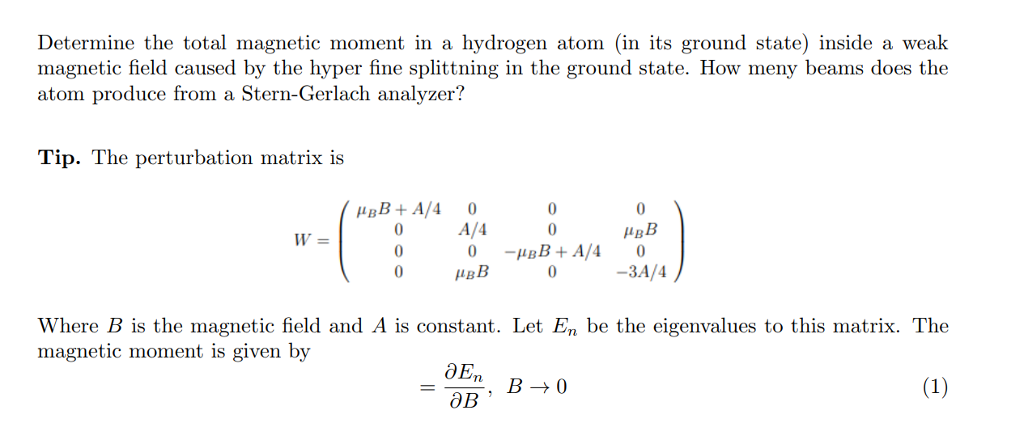
SOLVED:A hydrogen atom in a 3p state is placed in a uniform external magnetic field B . Consider the interaction of the magnetic field with the atom's orbital magnetic dipole moment. (a)
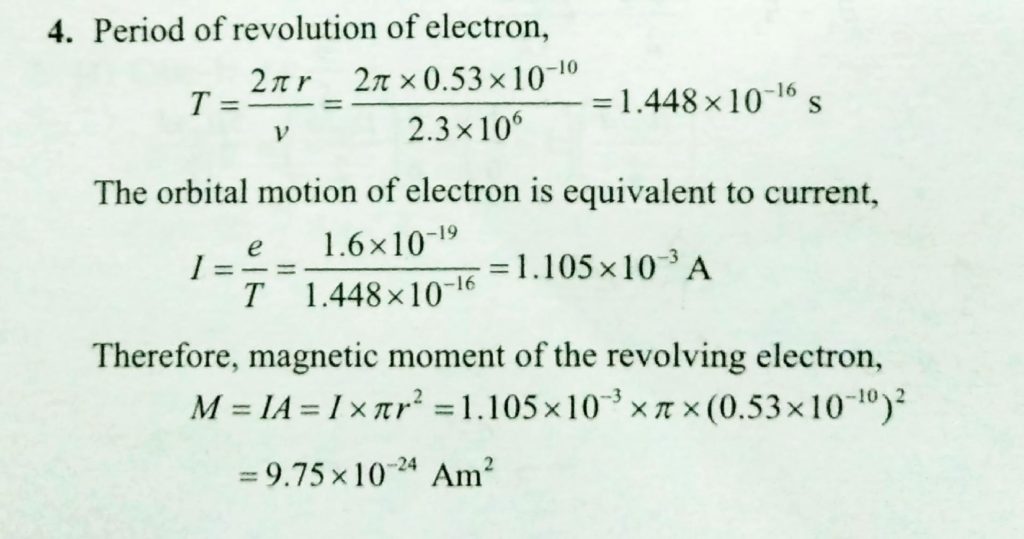
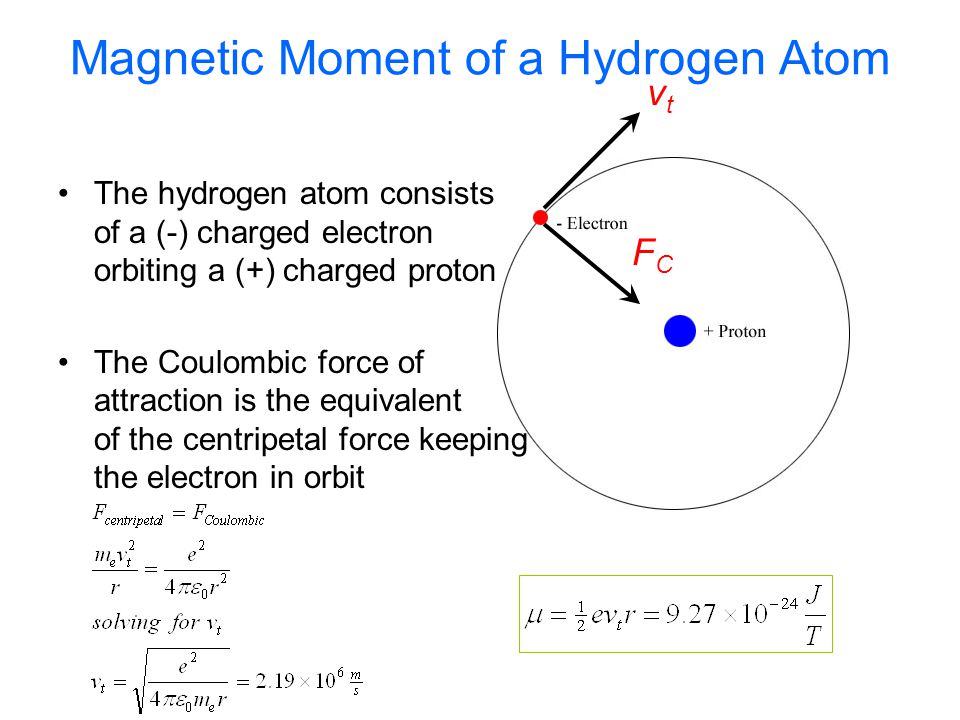
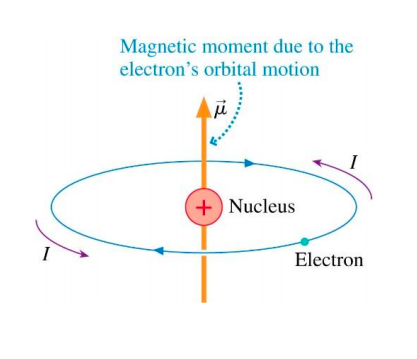
The electron in the hydrogen atom is moving with a speed of 2.3 x 10^6 m/s in an orbit of radius 0.53 A. Calculate the magnetic moment of the revolving electron. - Sahay LMS
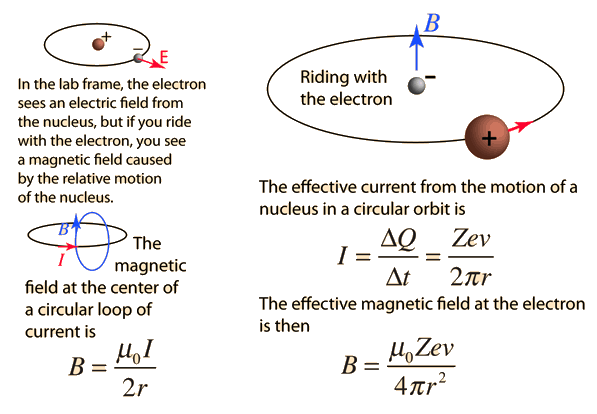
In a hydrogen atom, an electron of charge revolves in an orbit of radius r with speed v. What is the magnitude of the resulting magnetic moment of the electron? - Quora

Physics - Ch 66.5 Quantum Mechanics: The Hydrogen Atom (34 of 78) Magnetic Moment in Hydrogen - YouTube

quantum mechanics - Ignoring spin, what is its orbital magnetic moment of an electron in a hydrogen atom in the 2p orbital? - Physics Stack Exchange

10 EXAMPLE Magnetic dipole moment of a revolving electron An electron revolving in an orbit of radius 0.5 À in a hydrogen atom executes 10 revolutions per second. Find the magnetic moment
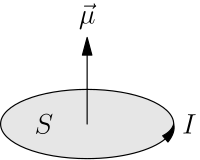
Prove that the magnetic moment of the electron revolving around anucleus in an orbit of radius r with orbital speed v is equal to evr/2.Hence using Bohr's postulate of quantization of angular

In the Bohr model of the hydrogen atom, the electron circuulates around the nucleus in a path of radius 5xx10^

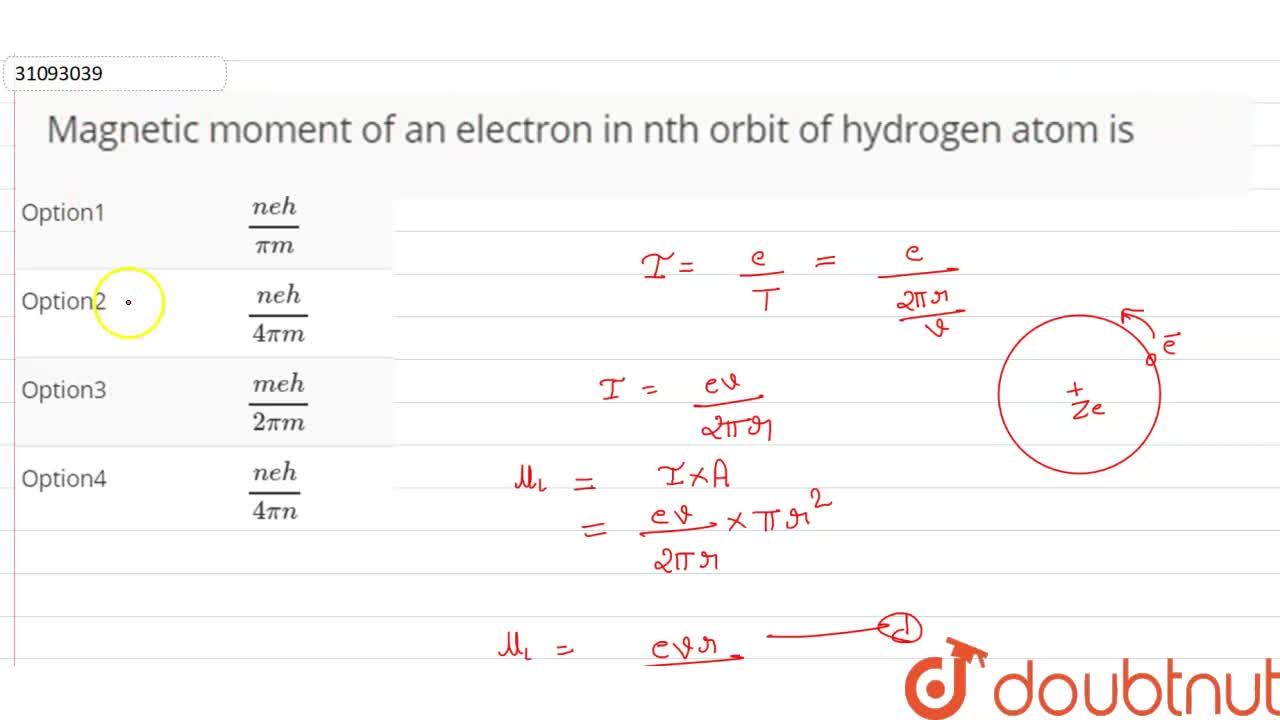
In the Bohr model an electron moves in a circular orbit around the proton. Considering the orbiting electron to be circular current loop, the magnetic moment of the hydrogen atom, when 5th