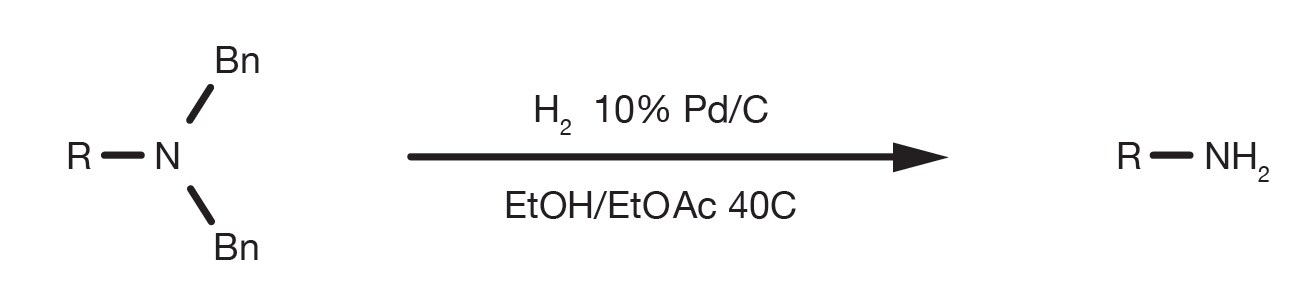
A Mild Deprotection Strategy for Allyl-Protecting Groups and Its Implications in Sequence Specific Dendrimer Synthesis

Molecules | Free Full-Text | Nickel-Catalyzed Removal of Alkene Protecting Group of Phenols, Alcohols via Chain Walking Process | HTML
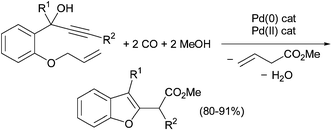
Sequential homobimetallic catalysis: an unprecedented tandem Pd(0)-catalysed deprotection – Pd(ii)-catalysed heterocyclisation reaction leading to benzofurans - Chemical Communications (RSC Publishing)

Facile Hydrogenative Deprotection of N-Benzyl Groups Using a Mixed Catalyst of Palladium and Niobic Acid-on-Carbon. - Abstract - Europe PMC
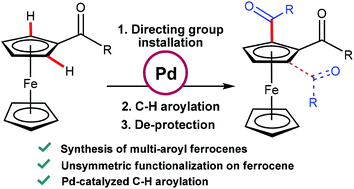
Synthesis of unsymmetrical multi-aroyl derivatives of ferrocene using palladium catalysed oxidative C–H aroylation - Dalton Transactions (RSC Publishing)
A Mechanistic Study of Direct Activation of Allylic Alcohols in Palladium Catalyzed Amination Reactions
Intracellular Deprotection Reactions Mediated by Palladium Complexes Equipped with Designed Phosphine Ligands
Intracellular Deprotection Reactions Mediated by Palladium Complexes Equipped with Designed Phosphine Ligands
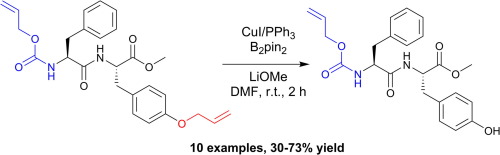
A mild copper catalyzed method for the selective deprotection of aryl allyl ethers,Tetrahedron Letters - X-MOL

SciELO - Brasil - Recent Progress in the Use of Pd-Catalyzed C-C Cross-Coupling Reactions in the Synthesis of Pharmaceutical Compounds Recent Progress in the Use of Pd-Catalyzed C-C Cross-Coupling Reactions in the
Optimised Conditions for the Palladium-Catalyzed Hydrogenolysis of Benzyl and Naphthylmethyl Ethers: Preventing Saturation of Aromatic Protecting Groups | Organic Chemistry | ChemRxiv | Cambridge Open Engage

Palladium-catalysed carboformylation of alkynes using acid chlorides as a dual carbon monoxide and carbon source | Nature Chemistry

Figure 5 from Palladium-triggered deprotection chemistry for protein activation in living cells. | Semantic Scholar

Intracellular Deprotection Reactions Mediated by Palladium Complexes Equipped with Designed Phosphine Ligands. - Abstract - Europe PMC